Journal Policy
Ethical Issues
Manuscripts submitted to the journal must be original, previously unpublished and not under the consideration of other journals or proceedings/conferences planning to publish in a special issue of MAB simultaneously. Authors are responsible to obtain written permissions to reproduce any materials from other sources that are used in the manuscript, e.g. figures, tables and illustrations.
Open Access
The journal is an open access journal which is freely available online. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of these articles without prior permission from the publisher and authors.
Copyright & Licensing
The publisher holds the copyright of the published articles. Any reproduction of figures, tables and illustrations must obtain written permission from the Chief Editor (wicki@ukm.edu.my). No part of the journal may be reproduced without the editor’s permission. The journal is published by:
The Malaysian Society of Applied Biology
c/o Department of Biological Sciences and Biotechnology,
Faculty of Science and Technology,
Universiti Kebangsaan Malaysia,
43600 UKM Bangi, Selangor, Malaysia.
Malaysian Society of Applied Biology (MSAB) does not hold itself responsible for statements made in the journal by contributors. Unless so stated, contents of the journal do not reflect an endorsement or an official attitude of MSAB or the Editorial Board.
Publication Ethics and Malpractice Statement
Malaysian Applied Biology (MAB) Journal is a Scopus indexed journal committed to safeguard the highest standards of publication ethics. The parties who are involved in the act of publishing (authors, reviewers, editors and publisher) have to follow the standards of publication ethics. MAB Journal publication ethics and malpractice statement is based on the Code of Conduct and Best Practice Guidelines for Journal Editors of the Committee on Publication Ethics – COPE (http://publicationethics.org/).
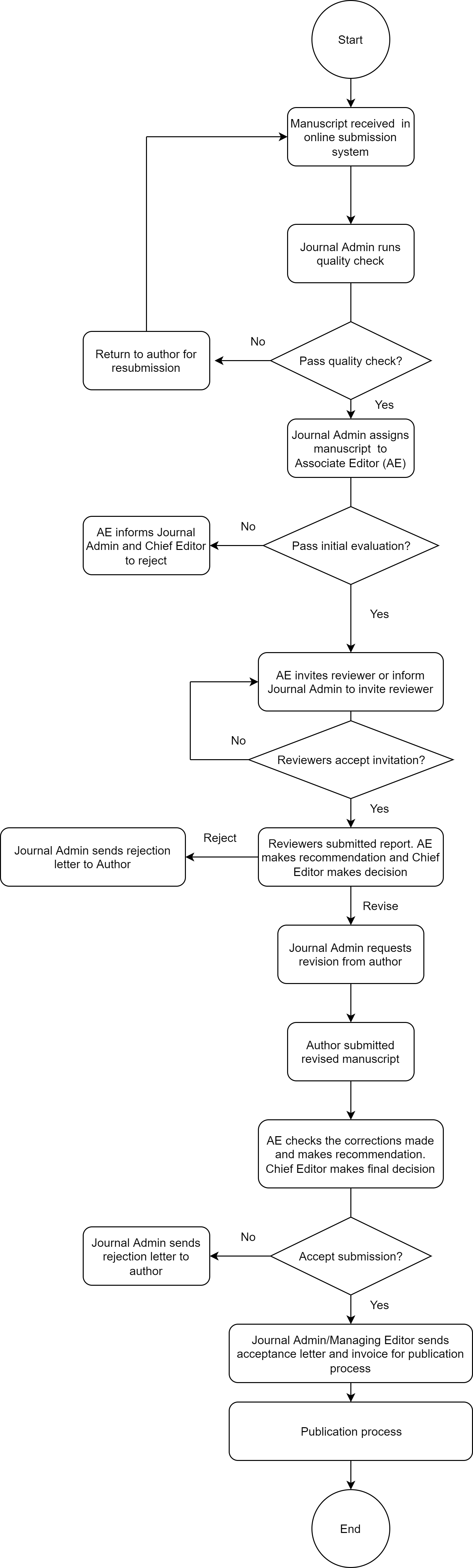
All manuscripts are initially evaluated by the journal admin for plagiarism and formatting. Manuscripts with more than 25% similarity and not complying with the instructions can be returned or rejected. Manuscripts with less than 25% similarity (check using Turnitin) and complying with the instructions to authors will be sent out to one of the Editors for more evaluations. Manuscripts rejected at this stage lack sufficient originality, contain major scientific problems, contain errors in the grammar or English language, or fall outside the journal's aims and scope. Those that meet the minimum criteria are typically forwarded for assessment to at least two professional reviewers. Rejection of manuscripts at this stage usually is notified within two weeks of receipt.
Type of peer review
Malaysian Applied Biology journal practices ‘double blind’ reviewing, which means the author(s) remains anonymous to the reviewers and the reviewers’ identity remains anonymous to the author(s). Reviewers are selected according to their expertise which is matched the submission.
- MAB editors are responsible to evaluate submitted manuscripts based on its merit and relevance to the journal scope without considering authors gender, race, ethnic origin, sexual orientation, religious belief, citizenship, institutional affiliation or political philosophy. The Editor-in-Chief has full authority to decide the editorial content and timing of publication of issue.
- Editorial board members will not disclose any information to anyone except potential reviewers.
- Editorial board members that have acquired information or ideas as a result of handling the manuscript will be kept confidential and will not use it for their personal advantage. Editors having conflict of interest resulting from competitive, collaborative or other relationships/connections with any of authors, companies or institutions connected to the papers will request for another member of the editorial board to handle the manuscript.
- Editorial board will ensure that all submitted manuscripts being considered for publication undergo peer-review by at least two reviewers who are experts in the said field.
- Editor-in-Chief will make editorial decision of each submitted manuscript based on reviewers’ comments, quality of work and importance to researchers, readers and such legal requirements as are currently in force regarding libel, copyright violation and plagiarism. The Editor-in-Chief may discuss with respective associate editors or reviewers in making this decision.
- Any ethical concerns that were raised with regards to a submitted manuscript or published paper will make the MAB editorial team in conjunction with publisher to look into these reported act of unethical publishing behaviour seriously and take actions even if it is discovered years after publication. MAB editors will follow the COPE Flowcharts (https://publicationethics.org/guidance/Flowcharts) when dealing with cases of suspected misconduct. If on investigation, the ethical concern is understandable, a correction, retraction, expression of concern or other note as may be relevant, will be published in the journal.
- Reviewers are responsible to assist editors in making editorial decisions and may assist authors in improving their manuscripts through editorial communications.
- Reviewers are responsible to notify MAB editors immediately after receiving the manuscript from MAB, if they feel unqualified to review the research reported in the manuscript or rapid review is impossible for them due to their time constraint.
- Reviewers who received manuscripts have to treat it as a confidential document and must not be shown to or discussed with others except if authorized by the Editor-in-Chief. This rule applies also to invited reviewers who decline the review invitation.
- Reviewers should conduct the review process objectively and should formulate observations clearly with supporting arguments so that authors can use them for improving the manuscripts. Personal criticism of the authors is not acceptable.
- Reviewers should make sure that any statement given by authors in the manuscript that is an observation, derivation and argument that has been reported in the previous publication should be accompanied by the relevant citation and they also should be able to identify relevant published work that has not been cited by the authors. During the review process, if a reviewer observed any similarity or overlap between the manuscript under consideration and any other manuscript published or unpublished of which they have personal knowledge should notify the editor immediately.
- Any invited reviewers who has conflict of interest resulting from collaboration or other relations or connections with any of the authors, institutions or companies connected to the manuscript are supposed to notify the editors immediately to declare their conflict of interest and decline the invitation to review so that alternative reviewers can be contacted. Invited reviewers who acquired information or ideas as a result of handling the manuscript will be kept confidential and will not use it for their personal advantage. This applies also to the invited reviewers who decline the review invitation.
- For original articles submission, authors should present an accurate amount of the work performed by them with results, followed by the discussion. Authors also need to incorporate sufficient detail and references to permit others to replicate the work. For review articles submission, the content should be objective, accurate and comprehensive. False or knowingly incorrect statements are considered as unethical and are unacceptable.
- During manuscript submission process, authors may be asked to provide the raw data of their work together with the manuscript for editorial review and should be prepared to make the data publicly available if practicable. Ensuring accessibility of data is the responsibility of the authors for at least 10 years after the publication date via a subject-based data repository, institution, or a data centre. This needs to be available provided the confidentiality of the participants can be protected and legal rights concerning proprietary data does not prevent their release.
- Authors should guarantee on writing and submitting their completely original work and should not use others work. If authors are using other words or statements, it should be appropriately cited. Publications that have been significant in defining the nature of the work reported in the manuscript should also be cited.
- Plagiarism will occur in number of reasons including passing off another author paper as the authors own paper, extracting results from research conducted by other authors and copying or summarising significant results from research conducted by without proper citation. All the above forms of plagiarism will consider as unethical publishing behaviour and is unacceptable.
- Authors are not allowed to publish the research work in more than one journal and submit the manuscript journal concurrently to more than one journal. The above action is considered as unethical publishing behaviour and unacceptable. Publication of some kinds of articles including clinical guidelines and translations in more than one journal is sometimes justifiable provided that, authors and editors of the concerned journal must agree for the secondary publication, must reflect the same data and interpretation of the primary document and the primary paper must be cited in the secondary publication.
- Those individuals who meet the below authorship criteria should be listed as authors in the manuscript. The authorship criteria include - (i) Give authorship only to those who made significant contributions to the conception, design, execution, data acquisition, or analysis/interpretation of the study; (ii) Those who contribute in drafting the manuscript or revising it critically for important intellectual content; (iii) all authors must see and approve the final version of the paper before submission to the journal. Those persons who do not meet the criteria for authorship but provided substantial contribution in terms of technical help, writing, editing and general support should be acknowledged in the "Acknowledgements" section upon getting written permission from them. The corresponding author should make sure that all co-authors meet the above mentioned authorship criteria to be included in the authors list and should get consent from all co-authors to submit the manuscript for publication.
- In the early stage of submission itself, authors should disclose any conflicts of interest including financial ones such as educational grants or other funding, honoraria, participation in speakers’ agencies, membership, employment, consultancies, stock ownership, or other equity interest, and paid expert testimony or patent-licensing arrangements, as well as non-financial ones which include personal or professional relationships, affiliations, knowledge or beliefs in the subject matter or materials discussed in the manuscript. All sources of financial support for the work should be disclosed (including the grant number or other reference number if any).
- Authors should guarantee that they have properly acknowledged the work of others and cite the publications that have been significant in defining the nature of the work. Any new information obtained from other private sources through conversation, correspondence or discussion among third parties should be reported after getting their written consent to add these information in the manuscript. Authors should not use any sort of information or ideas obtained during handling some of the confidential services include reviewing manuscripts or grant applications, unless they get written consent from the respective authors who were involved in the service.
- Authors whose work involves using unusual hazardous equipment, procedures or chemicals, must state clearly in the manuscript. Those authors’ work which involved participating human volunteers or handling animals should perform in compliance with relevant laws and institutional guidelines and should mention a statement in the manuscript that informed consent and ethical approval was obtained for experimentation with human participants or animals. The privacy rights of human participants must always be followed.
- Authors are responsible to participate in the peer review process and work together fully with the editorial members by responding promptly for the requisition of raw data, proof of ethics approval, clarifications, patient consent form and copyright permissions. After the editorial decision made by the editors, authors should respond to the reviewers’ and editorial comments systematically, point by point, and in a timely manner, and make sure to submit the revising manuscript to the journal within the given deadline.
- When authors realise significant errors or inaccuracies in their own published work, it is their responsibility to quickly inform the journal’s editors or publisher and cooperate with them to either correct the paper in the form of a printing or writing or to retract the paper.
- If the editors or publisher discover from a third party that a published work contains a significant error or inaccuracy, then it is the authors’ responsibility to quickly correct or retract the paper. Authors can also provide evidence to the journal editors to support the correctness of the paper.
- Publisher in collaboration with editors, should take necessary steps to identify and prevent the publication of manuscripts where research misconduct has occurred and should not knowingly allow or encourage such misconduct to take place. Publishers should work closely with the editors to clarify situation and take necessary actions for those authors who suspected or verified the scientific misconduct, plagiarism or carried out fraudulent activities to publish the manuscript.
- Publisher is responsible for the preservation of scholarly research and provides accessibility by affiliating with organizations and maintaining own digital archive.
Special Issue
Special Issue articles should fulfill all the normal requirements of any Malaysian Applied Biology article and should be of relevance to all fields of biology and applied biology or related scientific field. Authors should note that the same criteria of quality, originality and significance apply to articles in Special Issues as to regular articles. Special Issue articles must not consist of overviews of the author’s previously published work.
MAB publishes special issues for selected papers from conferences/symposium/seminar and special issues on selected or interesting topics. (MAB has stopped accepting papers from conferences/symposium/seminar effective 2024)
Special Issues on selected topics are led by Guest Editors who are experts in the subject and responsible to conduct the evaluation and editorial process. Guest Editors are appointed by MAB Editorial Board.
All special issues from a conference/symposium/seminar must receive initial approval from the MAB Editorial Board before the papers are submitted. The proposal should be emailed to the Chief Editor. The conference committee should sign an agreement with MAB and should inform the tentative date of submission of special issue manuscripts to MAB. - Stopped since 2024.
The conference committee should suggest a number of Guest Editors for the special issue. MAB Editorial board has the right to decline the suggestion (based on CV and reputation) and request the committee to suggest new editors.
- Manuscripts should be prepared according to Instructions to Author (https://jms.mabjournal.com/index.php/mab/instructions_to_author)
- Each author is allowed to submit a maximum of two manuscripts in their name (as first author/co-author/corresponding author) for this special issue.
- The reviews will be conducted using the online submission portal (https://jms.mabjournal.com/index.php/mab)
- After receiving the manuscripts, the MAB Editorial Board will assign a manuscript ID to each manuscript and perform the initial quality check. The Editorial Board has the right to reject the manuscript on basis of quality, significance, scope, and plagiarism (Turnitin percentage should be ≤ 25%).
- The guest editors will be given access to the system and can start processing the manuscripts.
- The appointed guest editors are responsible to send out the manuscripts to at least two independent reviewers for each manuscript.
- The review process must be a double-blind peer review (authors and reviewers should not know each other’s names and affiliations).
- After receiving the comments from reviewers, the guest editors should make a recommendation and send the comments to the authors for revision.
- Authors should upload the revised manuscript to the portal. The guest editors should ensure the revision is adequate and authors have responded to the reviewers’ comments/suggestions. Guest editors need to send the recommendation of the manuscript to the Editorial Board for acceptance. The Editorial Board has the right to ask for further revision or reject the manuscripts if the authors did not respond to the comments/suggestion.
- If the review process was conducted outside of the portal and before the formal approval of the special issue from the Chief Editor, the review reports and author’s responses to the reviewers’ comments must be uploaded to the submission portal together with the revised manuscripts. The Editorial Board has the right to ask for further revision.
Research and Publication Ethics
Studies involving vulnerable populations (i.e., children, elderly, disabled persons, etc.,) would be subjected to additional checks and evaluations. Consent must be obtained from subjects in these populations and the Editorial Board may request evidence such as the blank consent form and any documents related to the process of getting the ethical committee approval. Authors must clearly state the procedure for conducting the studies and must strictly adhere to national or institutional regulations. Authors should also state the approval code or reference and the name of the ethics committee in the Ethical Statement section. Publication involving human participants must obtain informed consent from the participants or their parent or legal guardian in the case of children under 16 and a statement to this effect should be displayed in the manuscript. Protection of patient anonymity should be prioritized especially for manuscripts reporting studies involving vulnerable groups (for example, unconscious patients) where there is the potential for coercion (for example prisoners). Consent must be obtained for all forms of personally identifiable data including biomedical, clinical, and biometric data. In the case of articles describing human transplantation studies, authors must include a statement declaring that no organs or tissues were obtained from prisoners and must also name the institution(s)/clinic(s)/department(s) via which organs/tissues were obtained. Documentary evidence of consent must be supplied if requested. Images without appropriate consent will be removed from publication.
We recommend the author refer to the national or institutional guidelines for conducting and reporting studies on these populations.
1. https://bioethicsarchive.georgetown.edu/nbac/human/overvol1.pdf
2. https://methods.sagepub.com/reference/the-sage-encyclopedia-of-communication-research-methods/i15598.xml)
Involvement of Animal or Human Subjects
Authors must follow the ethical guidelines for the use of animal or human subjects in their research.
MAB recommends the ARRIVE guidelines (arriveguidelines.org/) for reporting studies using live animals. Authors and reviewers must adhere to the ARRIVE guidelines (https://arriveguidelines.org/sites/arrive/files/documents/ARRIVE%20Compliance%20Questionnaire.pdf). Failure to adhere to the guidelines would result in the rejection of the manuscript. Authors are required to state relevant information regarding the welfare of the animal, husbandry and housing.
All studies involving human subjects, human material, or human data must adhere to the Declaration of Helsinki and be approved by an authorized ethical committee. The authors must confirm that informed consent was acquired from all subjects.
Authors are required to obtain formal approval from an appropriate ethical committee before conducting studies and carrying out the research in accordance with national and institutional regulations. Authors should state the ethical committee approval details; project number/code, approval date and the name of the ethics committee in the Ethical Statement section. In a situation where ethical approval is not required, the authors must supply the exemption proof and provide a relevant statement or explanation of the exemption in the Ethical Statement section.
Clinical Trials
All clinical trials must be registered in a public registry prior to submission. The trial registry number must be included in the manuscript. Authors will be expected to have obtained ethics committee approval and informed patient consent for any experimental use of a novel procedure or tool where a clear clinical advantage based on clinical need was not apparent before treatment. The journal follows the trials registration policy of the International Committee of Medical Journal Editors (ICMJE, www.icmje.org). Acceptable registries must meet the following ICMJE requirements:
- be publicly available, searchable, and open to all prospective registrants
- have a validation mechanism for registration data
- be managed by a not-for-profit organization
Examples of registries that meet these criteria include:
- ClinicalTrials.gov the registry sponsored by the United States National Library of Medicine
- The European Clinical Trials Database
- The International Standard Randomized Controlled Trial Number Registry
Randomised Controlled Trials (RCTs) must adhere to the CONSORT statement (CONsolidated Standards of Reporting Trials) and submissions must be accompanied by a completed CONSORT checklist (uploaded as a related manuscript file). Please see www.consort-statement.org for further information
Publication timeline
Our goal is to inform the authors of the decision on their manuscript(s) within two months after submission. However, it depends largely on the quality of the manuscript and the response of authors and reviewers. It will take a longer time due to the following reasons: 1) The editor needs to seek a third opinion due to conflicting reviewer reports; 2) The reviewer/s requested a resubmission if the reviewer/s thinks that the manuscript can be further improved; 3) The author/s did not make all of the suggested changes within in the given timeframe.